Hydrochloric Acid Formula - Hydrochloric Acid Uses, Properties, Structure and Formula
Hydrochloric acid is the aqueous solution of hydrogen chloride. It is a strong mineral acid with many industrial uses.
Formula and structure: The chemical formula for hydrochloric acid is HCl, and its molecular weight is 36.47 g/mol. It is the solution of hydrogen chloride in water, and HCl is used synonymously for both the gaseous form and the aqueous solution. HCl is a simple diatomic molecule, with a polarized covalent bond between the hydrogen atom and the electronegative chlorine atom.
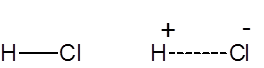
Occurrence: Hydrochloric acid is the main constituent of natural gastric acids in our stomach.
Preparation: It is prepared by dissolving hydrogen chloride in water. Hydrogen chloride is typically generated as the by-product from industrial scale production of other chemicals. It is also prepared industrially by the combustion of hydrogen in chlorine. High concentrations of HCl are difficult to prepare due to evaporation.
Physical properties: Hydrochloric acid is a clear, colorless solution and has a highly pungent odor. It is available in many different concentrations in water, thus its exact physical properties (boiling point, melting point and density) vary accordingly.
The concentrated grade (fuming hydrochloric acid) is about 38% HCl in water. Industrial-grade HCl is about 30% to 35%, while the commercial grade (muriatic acid) is between 20% and 32%. Household cleaning solutions of HCl are typically 10% to 12%, but these still need further dilution before use.
Chemical properties: HCl is a strong, monoprotic acid, which means it can release only one H+ ion (proton). Being a strong acid, it gets completely dissociated in water to give the hydronium and chloride ions. It readily reacts with bases to form chloride salts. Concentrated HCl dissolves many metals and forms oxidized metal chlorides and hydrogen gas. Dilute HCl can break down or digest many chemical and biological samples.
Uses: Hydrochloric acid has many industrial uses, such as in the production of various chlorides, plastics (such as PVC and polyurethane), fertilizers, and dyes. It is also used in the photographic, textile, and rubber industries, as well as in gelatin production, leather processing and household cleaning products.
Health hazards/ health effects: HCl is very corrosive to the eyes, skin, and mucous membranes. Skin contact results in severe burns and scarring. Inhalation of the fumes can cause irritation of eyes, nose, and respiratory tract. Ingestion of the acid causes tissue damage to the mucous membranes, esophagus, and stomach.
|
Related Links: |
